Most hernias today are treated using surgical mesh. However, over the past decade an increasing number of patients have suffered severe complications caused by defective hernia mesh products.
If you experienced adverse side-effects following hernia surgery, or if you required revision surgery to remove and replace a hernia mesh patch, you may be entitled to compensation. Please call the Oklahoma product liability lawyers at Carr & Carr Attorneys at Law today at 866-510-0580 or contact us online for a free consultation.
We have offices in Tulsa and Oklahoma City, but our attorneys work with defective product victims nationwide. Consultations are complimentary, and we don’t charge for our services unless we recover compensation on your behalf.
Types of Hernias Treated with Surgical Mesh
Hernias occur when an internal organ is pushed through a tear or gap in the surrounding muscle or tissue, which often results in a visible bulge in the affected area. Hernias can affect men or women, and they are most common in the lower abdomen or groin.
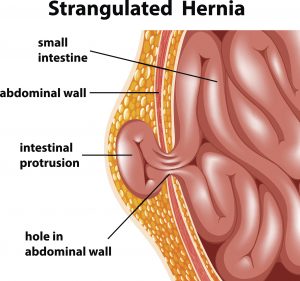
Hernia repair is typically accomplished by surgically placing a mesh patch over tears in the weakened muscle or tissue. The mesh—which is created from a medical-grade biological or synthetic material—acts as a barrier to prevent organs from breaking through again.
Surgical mesh is used to treat many types of hernias, including:
- Inguinal hernias, which occur near the inner groin
- Femoral hernias, which occur in the upper thigh/outer groin region
- Ventral hernias, which occur along the midline of the abdomen
- Hiatal hernias, which occur in the upper stomach or diaphragm area
- Umbilical hernias, which occur around the belly button
- Incisional hernias, which occur in the areas around previous abdominal incisions
Hernias historically have a high recurrence rate, and one of the reasons surgical mesh has become favored for hernia treatment is that it generally reduces the risk of recurrence. But in recent years, a number of hernia mesh products have proven ineffective and actually contributed to the risk for post-repair complications and the need for revision surgery.
Defective Hernia Mesh Implants
One possible contributing factor to the rise in hernia mesh complications is the approval process employed by the Food and Drug Administration (FDA) for certain medical devices.
New prescription medications and medical devices must undergo thorough clinical testing and meet other rigorous standards before they are approved by the FDA as safe and effective for broad use. But the FDA also maintains the so-called 510(k) process, which allows manufacturers of products that are “substantially equivalent” to other FDA-approved devices to skip clinical trials.
The safety of several surgical mesh implants approved through the FDA’s 510(k) process has been questioned following thousands of reports that the devices have caused post-surgery complications. Some surgical mesh makers, including C.R. Bard and Johnson & Johnson subsidiary Ethicon, have already recalled surgical mesh products and collectively paid more than $1 billion to settle lawsuits stemming from complaints that the manufacturers were aware of their products’ risks and failed to warn surgeons and consumers of their potential complications.
Meanwhile, the FDA has received additional complaints about hernia mesh products including Atrium’s C-QUR and Ethicon’s Physiomesh, which were granted 510(k) premarket clearance. Although some hernia mesh products have been recalled or withdrawn from the market due to potential risks, others are still in use, and many hernia patients may still have faulty mesh products that have not yet caused complications.
Hernia Mesh Complications and Symptoms
There are a number of complications that can arise from defective surgical mesh, including:
- Infection
- Mesh adhesion to internal organs
- Mesh migration
- Mesh obstruction of the bowels or bladder
- Perforation of surrounding tissue or organs, and internal bleeding

Symptoms of faulty hernia mesh include:
- Chronic pain that may be centered around the surgical site but extend through the abdomen, groin and legs
- Fever and/or chills
- Rashes around the surgical site
- Sudden nausea
- Difficulty urinating or having bowel movements
- Onset of constipation or diarrhea
- Hernia recurrence
Hernia mesh complications generally require a revision surgery to remove the mesh and retreat the hernia itself. If not treated in a timely fashion, some of these complications can be life-threatening.
Hernia Mesh Manufacturers Facing Lawsuits
Manufacturers currently facing legal action over their hernia mesh products include:
- Atrium (C-QUR mesh products)
- C.R. Bard (PerFix, Kugel, 3Dmax, Ventralex, Sepramesh and Composix mesh products)
- Covidien (Parietex mesh products)
- Ethicon (Physiomesh, Proceed and Prolene mesh products)
If you or a loved one underwent hernia repair with a surgical mesh product and later experienced complications, it’s advisable to discuss your case with a knowledgeable product liability attorney. You may be eligible to recover compensation for damages including medical expenses, lost wages, and pain and suffering.
Experienced Legal Help for Hernia Mesh Victims
At Carr & Carr, our attorneys are dedicated to helping victims of faulty medical devices recover the financial security they need to move forward with their lives.
We offer free consultations to help you understand your options, and our lawyers work on a contingent-fee basis, which means we don’t charge for our services unless we win your case. If you or a loved one suffered complications after hernia repair using surgical mesh, please call us at 866-510-0580 to schedule your personal consultation or contact us online to tell us your story now. Carr & Carr maintains offices in Tulsa and Oklahoma City, but we represent clients from across the country.

